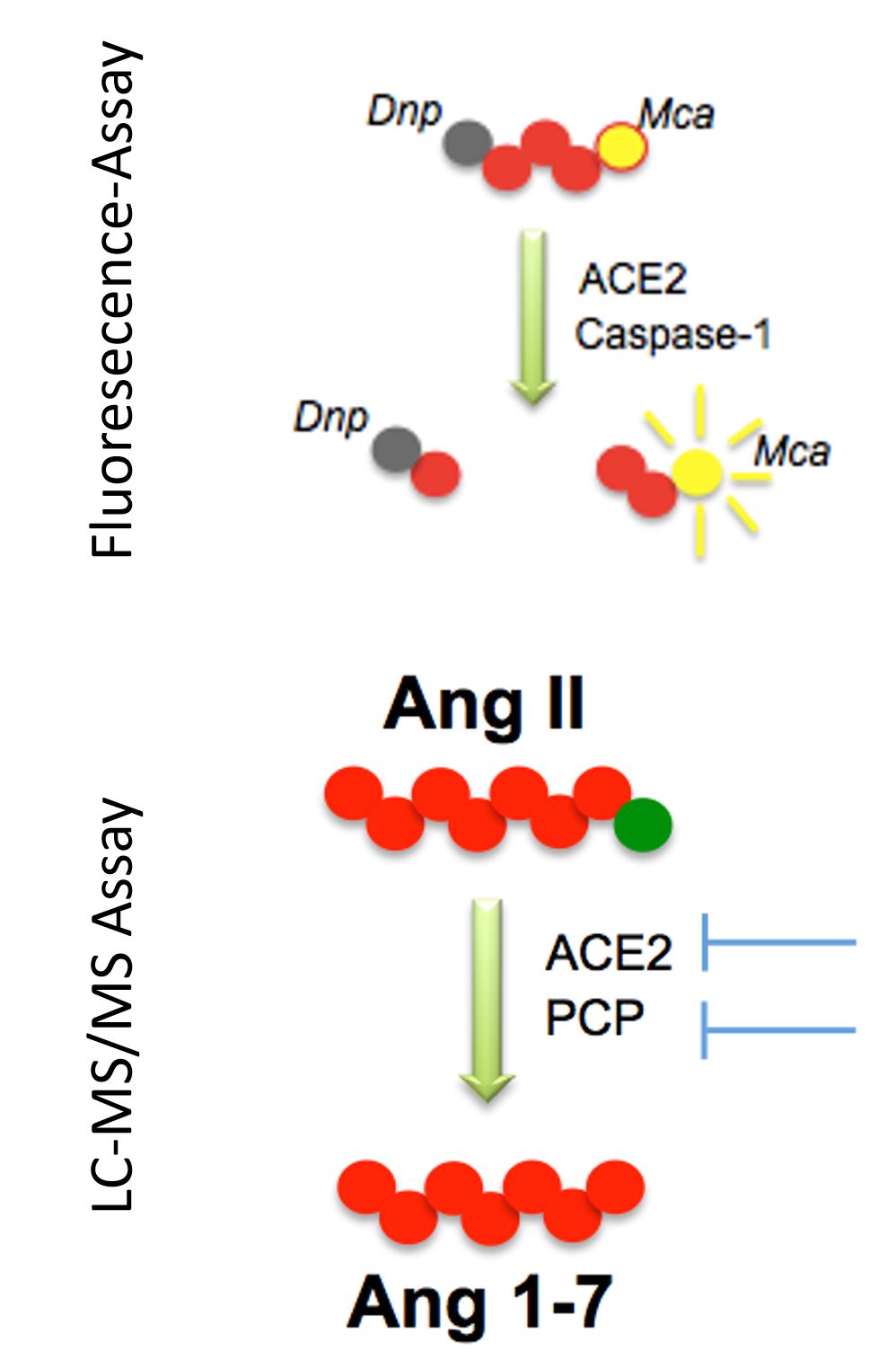
The measurement of protease activities in tissue and plasma samples is a challenging task as the importance of assuring signal specificity is widely underestimated. Common strategies aimed to determine the activity of proteolytic enzymes in complex samples employ the cleavage of artificial substrates. Colored or fluorescent products that are released from a quenched artificial substrate upon protease cleavage are measured and enzyme activities are quantified using a standard curve of recombinant enzyme that is usually prepared in matrix free buffers. Importantly, biological samples contain dozens of proteases that can cleave such artificial substrates, which usually contain very few amino acids that aiming to mediate the selectivity for a certain enzyme. As a consequence, especially low abundance enzymes cannot be accurately quantified in complex samples using commercially available colorimetric or fluorimetric kits.
Our LC-MS/MS based approaches combine the conversion of natural substrates to the natural product of a certain enzymatic reaction with the highly sensitive and direct LC-MS/MS based quantification of the enzyme specific product. The additional employed specific inhibitors guarantee the selectivity of the assay. Standard curves of recombinant enzymes are routinely prepared in the original sample matrix and signal specificity is further confirmed by the use of specific enzyme inhibitors