Sampling Requirements: The dynamic nature of the RAS with very short angiotensin metabolite half-lives down to seconds as well as rapid on-going angiotensin formation via renin are well known sources of contradicting angiotensin data in the literature. It is essential to thoroughly control the whole process from sampling down to signal detection, assuring the integrity and reproducibility of angiotensin data. This requires sampling in the presence of a special optimized angiotensin protease inhibitor cocktail (Circulating Angiotensin Levels) or adhering to sophisticated sample analysis protocols (Equilibrium Analysis, Tissue Analysis).
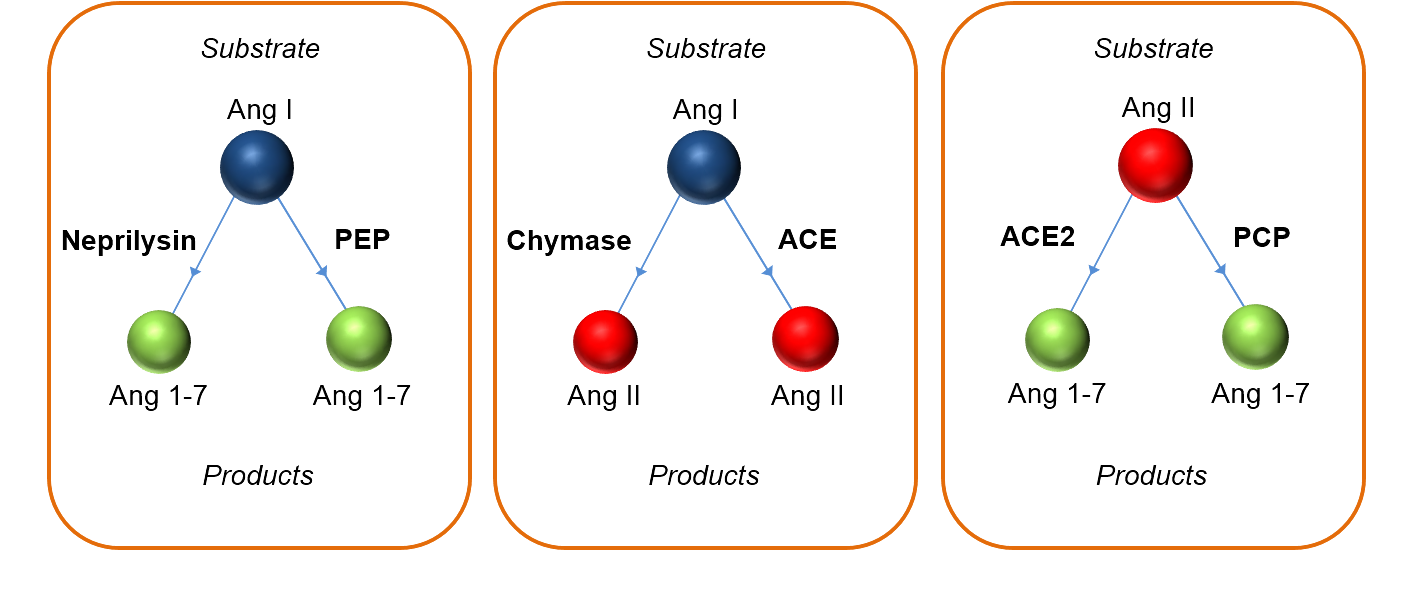
Angiotensin Sequence Similarity vs. Antibody Specificity: Different angiotensin metabolites share sequences with corresponding sections in their upstream metabolites including highly abundant angiotensinogen. Therefore, antibody cross-reactivity is a common issue for immunoassay based angiotensin quantification methods. Poor specificity and the incompatibility of these methods with internal standard technology is one of the main reasons why angiotensin data are often contradicting among different studies so far.
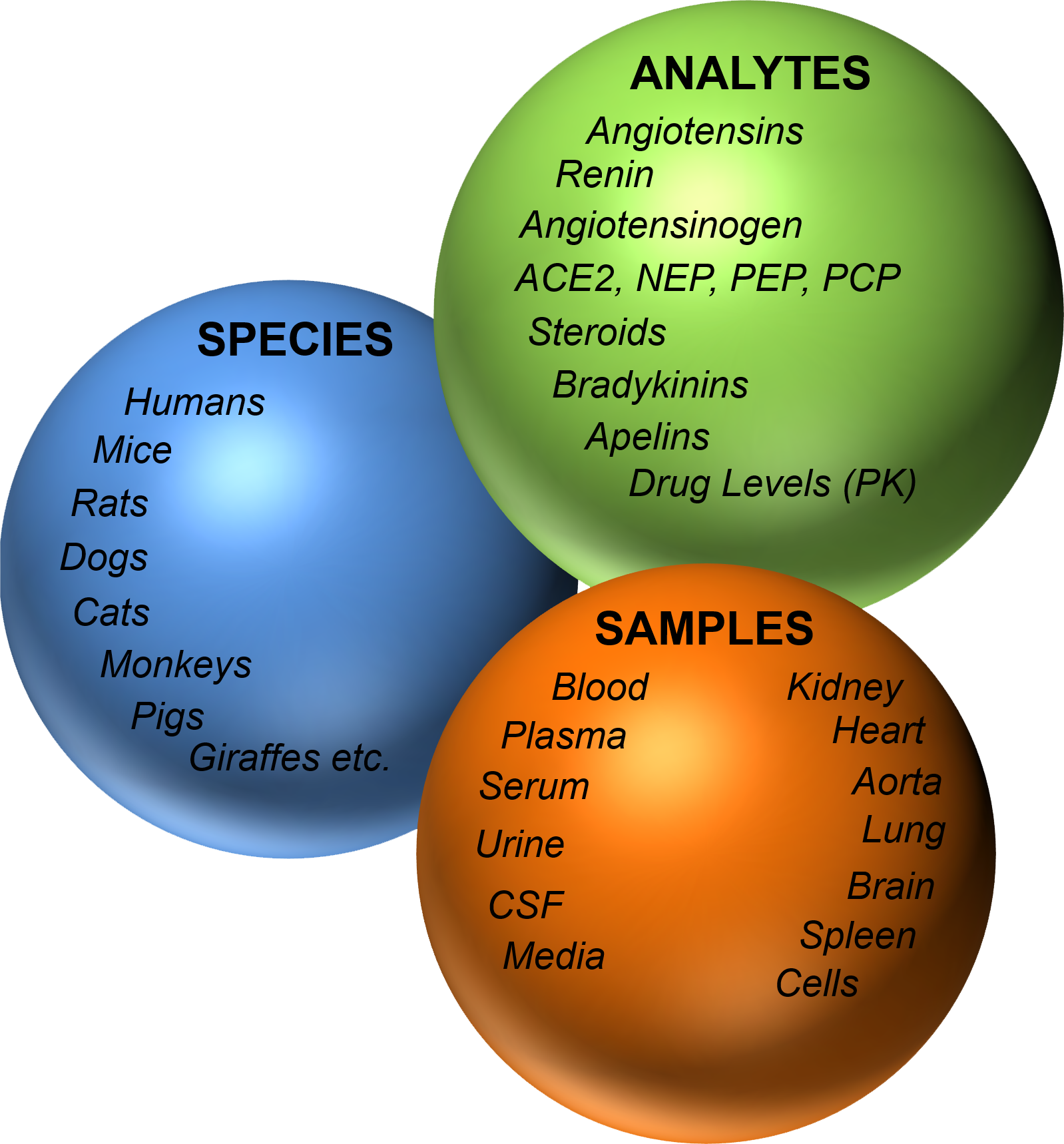
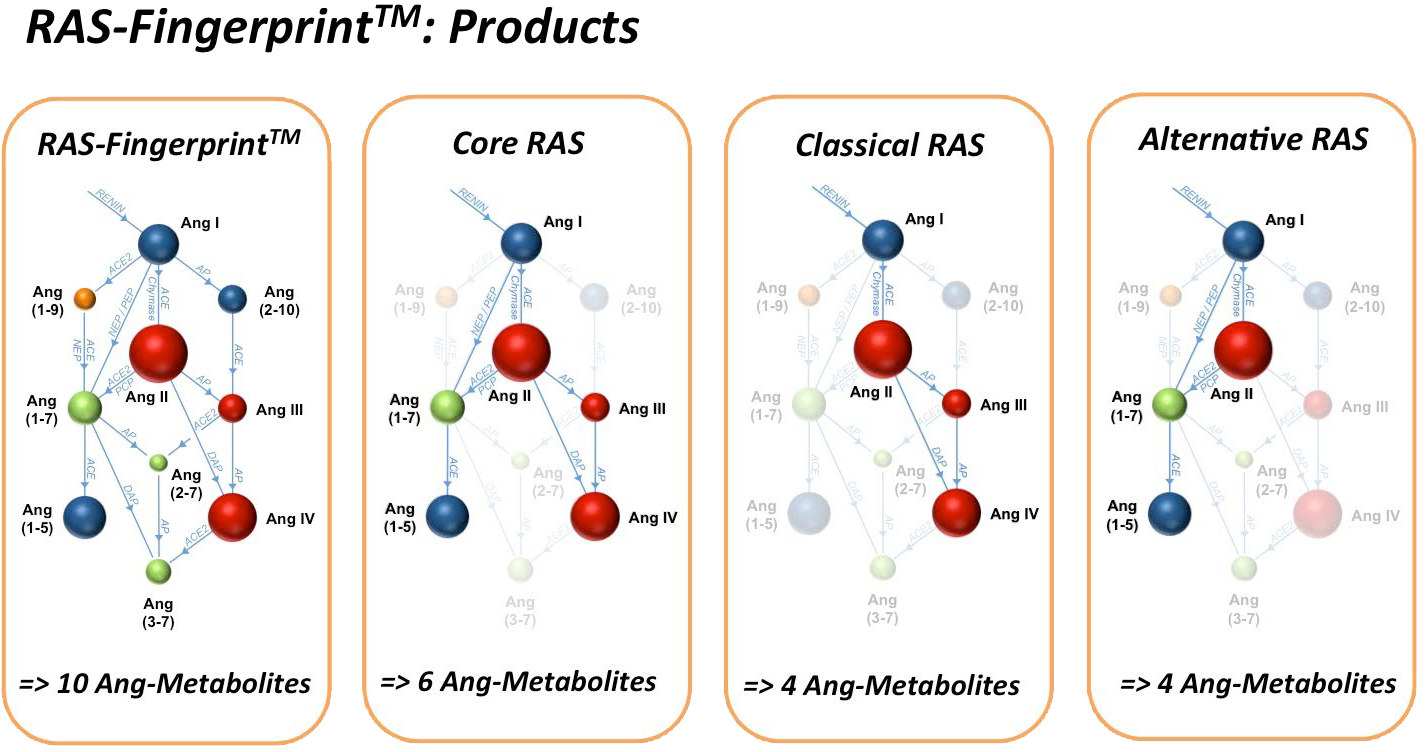
With the RAS-Fingerprint™, we provide a premium quality and full service solution to these issues, including consulting support for your study. Our expertise in RAS sample collection and processing in combination with high-end LC-MS/MS technology guarantee the highest analytic performance and quality standard for each individual sample. Angiotensin metabolite panels (RAS-Fingerprint™) depict additional information by visualizing the enzymatic connections between individual angiotensin metabolites. The graphs provide an attractive and unique way of displaying your data, thus facilitating the transfer of your findings to your targeted audience. Different functional RAS-Fingerprint™ panels are available for circulating plasma angiotensin levels, endogenous tissue angiotensin levels and plasma equilibrium levels.